Security
Patient first: choose quality, safety and expertise
01
Compliance and QM
LICENSED EGG AND SPERM BANK
Gametia complies with the European directives 2004/23/CE, 2006/17 /CE and 2006/86/CE, where the obtaining, donation, evaluation, traceability, storage and distribution of donor gametes are regulated), having obtained authorization as a human egg and sperm bank by the Spanish health authorities. Each individual site also complies with the local transposition of the European Directives:
- Spain: Spanish law of Assisted Reproduction 14/2006 and law 09/2014 on quality and safety standards for the donation, procurement, testing, processing, preservation, storage, and distribution of human cells and tissues , law 3/2018 on data protection.
- Italy: Italian law no. 40/2004 on Medically Assisted Procreation, Decree No 131/2019 regarding certain technical prescriptions relating to tissue examinations and human cells. , Decree No. 101/2018 on data protection.
Portugal: Law n.º 32/2006 about Assisted Reproduction , Regulation No. 1/2017 of the Authority for Blood and Transplantation Services (ASST) sets the technical and quality requirements for human cell and tissue banks. It specifies the conditions for the collection, processing, storage, and distribution of donated gametes, and law 58/2019 on data protection.
02
Technology
Our laboratories are governed by the highest quality standards. This is demonstrated both in our work processes and in the use of undisputed leading brands and technologies in the sector.
We have laboratories that are continuously updated, designed to obtain the best results, with the maintenance of gamete quality in mind. The distances to the operating room are minimal to guarantee the maintenance of physiological conditions, from follicular puncture to recovery by the embryologist. The laboratory has positive pressure and filtered air source to ensure high levels of sterility and minimization of VOC’s in the environment.
We firmly believe that only the best products and equipment deliver the best results.
These are the brands, and their products, that make us achieve excellence:
Our centers have this technology from Cooper Surgical, which is the only passive/automatic witness system, which minimizes the interaction of the embryologist and increases the safety and reliability of the entire process. All our processes are carried out with RI Witness, a commitment that provides us with 100% security and transparency for a receiving center, which will have the maximum confidence when it comes to acquiring samples. You will be sure to receive what you have requested; that we have chosen the right donor. In addition, you will have complete traceability of all the products used in the laboratory, batches, expiry dates, brands… R.I WItness allows us to offer you full transparency in our processes.
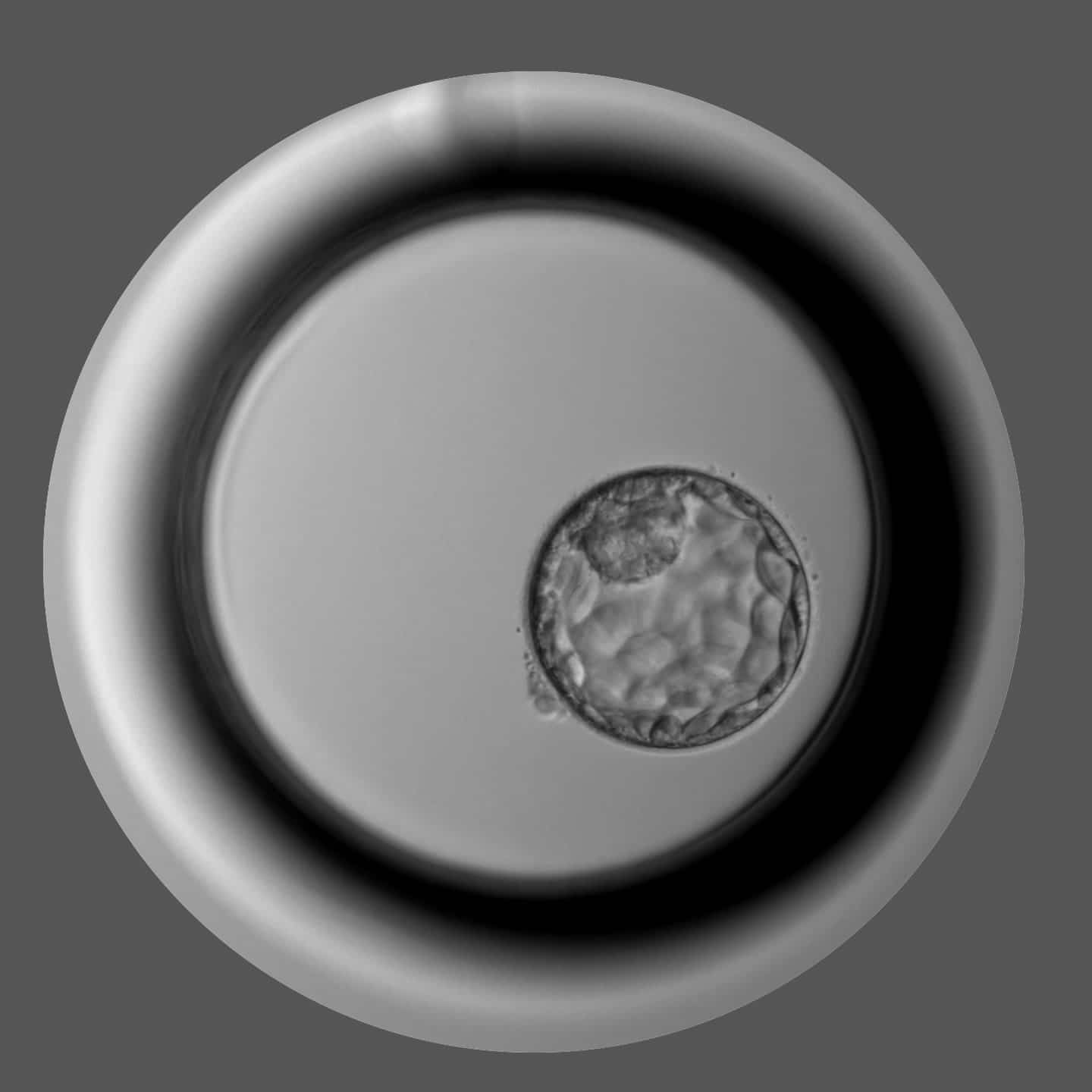
All the oocytes in our biobank are vitrified according to Kitazato’s Cryotop ® Method. We bet on this brand for several reasons. Kitazato is the gold standard in vitrification worldwide, with results that have been highly validated, both by publications and by recognized use. In this way we can guarantee, not only the best proven results in terms of survival rates, but we can offer you expert support to achieve these results.
All critical laboratory equipment is subject to our continuous monitoring thanks to Caltecnica’s services. We can control the temperature of the tanks and incubators, and the gases, set alerts for changes in variables for greater safety.


03
Traceability
3.1 Internal traceability – Witness
Gametia Biobank offers a guarantee of traceability and security in its processes with the use of RI Witness. Distance is not an impediment; Whether it is a double donation, or fertilization with sperm from the spouse, the gametes will be coded together, so that there is no confusion between the sperm and the oocyte chosen for fertilization.
3.2 Donor traceability – SIRHA
Gametia Biobank registers all its donors in the national registry of SIRHA (Assisted Human Reproduction Information System). We are fully committed to the traceability and transparency of donations. It is necessary to control the donor, to know the number of donations and live births.
3.3 Shipment traceability
On the first request, the customer sets the day. Based on this, a communication is established by mail and at the moment they are given the tracking number to be able to follow up, and the date of receipt is notified, which will vary depending on the destination. We work exclusively with the most reputable and reliable transport companies, ensuring that your shipment is fully covered for added security.
We are flexible and can adapt to your transport preferences. We can make shipments as a journey and delicate handling, hand by hand.