Shipments
Patient first: choose quality, safety and expertise

Logistics
Transportation is a fundamental part of our activity; we take care of every detail to guarantee a fast and safe delivery.
At Gametia we are experts in handling gametes and have access to different transportation options depending on the receiving centre preferences and individual requirements. We have performed more than 15.000 shipments per year.
Cells travel in certified dry shipper containers approved by IATA, which offer insulation and protection of the cells, and are protected by external shock resistant and leak proof cases built specifically for the transportation of biomaterials.
The dry Shipper is handled by an approved courier with authorisation to handle non dangerous biomaterial (UNE XXXXX). Within the container, depending on requirements we can place a temperature control probe with a data logger that reports any fluctuation in the internal temperature. If also needed, a tracking device can be added.
Delivery time is normally within 24-48h.
Dry Shippers autonomy: 120 hours
Cells travel in a certified dry shipper container approved by IATA, which offer insulation and protection of the cells, and are protected by external shock resistant and leak proof cases built specifically for the transportation of biomaterials.
This container is carried onboard by a trained and authorised courier , specialised for that service, who never looses sight of the package and delivers it directly to the receiving lab. Total traceability of the shipment is guaranteed by the courier operator. Within the container, depending on requirements we can place a temperature control probe with a data logger that reports any fluctuation in the internal temperature.
Delivery time 24-48h.
Dry Shippers autonomy: 120 hours
Available when distance allows it. Cells travel in certified liquid nitrogen containers approved by IATA that are protected by external shock resistant and leak proof cases built specifically for the transportation of biomaterials.
The containers are loaded in specially equipped vans (ventilation, anti- knock over restraints…) that is exclusively dedicated to the transport of our biomaterials.
The driver will deliver in hand the samples the receiving lab.
Within the container, depending on requirements we place a temperature control probe with a data logger that reports any fluctuation in the internal temperature. If also needed, a tracking device can be added.
Delivery time 1 to 5 days.
Liquid N2 Shippers autonomy: 10 days.
Irrespective of the chosen travel options the containers are sealed with a numbered fastener, anchored to the closure system. The identification number of the fastener is unique and is indicated in the shipping report.
All our shipments are controlled by our staff, controlling all the process from the departure to the delivery.
Upon arrival of the samples the receiving lab staff should check the cases, contents, probes and documentation and if everything is correct sign the delivery.
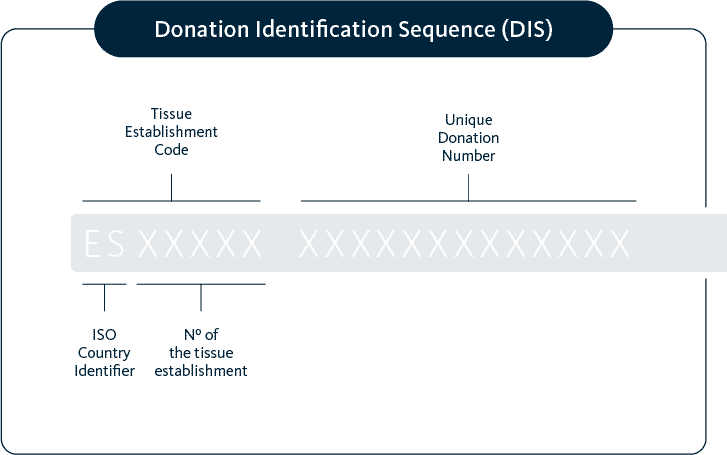
Single european code
and national registries.
All samples are codified and registered with a SEC code according to EU directives (2015/565) and local regulation. For example in the case of our Spanish biobanks the national SIRHA registry collects and manages the information of donors of gametes for reproductive purposes.
The SEC code makes easier the identification by the receiving Reproduction Center.
Donor transparency is one of the pillars of the legal framework and implies accessible and precise information about legal, ethical, and clinical aspects involving an effective control of new borns.
What is it for?
The Single European Code is an identifier which supports traceability (the tracking of the whole process of cells from the donor to the recipient) and provide information on the main characteristics of such cells.
It allows the user to obtain information on the cells and the establishment associated with them, including the activities for which he is authorized and their contact details, essential information for the end users.
the SEC Code
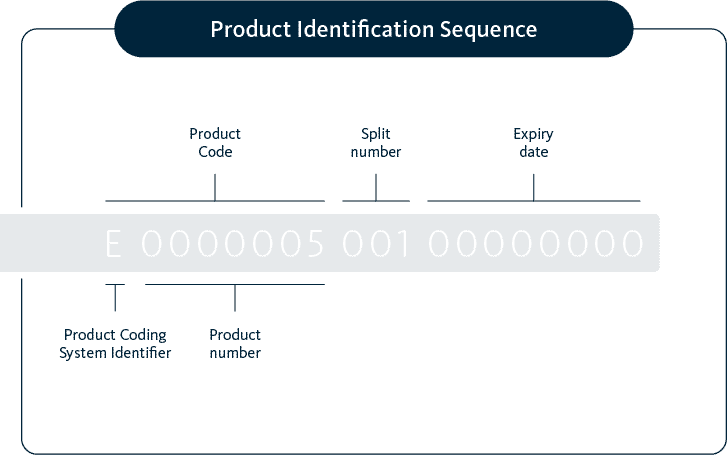
* Note:
- E0000056 for embryos
- E0000057 for oocytes
- E0000059 for sperm
What is it for?
The Single European Code is an identifier which supports traceability (the tracking of the whole process of cells from the donor to the recipient) and provide information on the main characteristics of such cells.
It allows the user to obtain information on the cells and the establishment associated with them, including the activities for which he is authorized and their contact details, essential information for the end users.
Donor transparency
is one of the pillars of the legal framework and implies accessible and precise information about legal, ethical, and clinical aspects involving an effective control of new borns.
Full control
All biological samples from Gametia biobank are obtained and processed in clinics under our full control, following the same medical and scientific criteria. Our biobank exclusively recruits donors in Spain and Portugal, declining to use agencies for recruitment purposes in any other countries.
Some Biobanks frequently import donor samples from NON EU countries, like Ukraine, into Eastern EU countries and then, exploiting a poor compliance enforcement, they assign to the sample an EU SEC code from a clinic that was not involved in obtaining the samples from the donors. This practice bypasses EU regulations to enable an parallel distribution within the EU, while posing risks due to loss of traceability and potential ethical and quality issues. Due to lack of accountability and clinical documentation in the country of origin and the change of codes in the EU importing centers, the final receiving centers cannot rule out unethical recruitment and treatment of donors, compliance irregularities, and most importantly health and safety concerns for the patience as proper Biosurveillance is forfeited in the process. The representatives of the centers where these samples are utilised will be held accountable by their local health authorities.
