Introduction
For decades, cryopreservation of human semen in Assisted Reproduction Techniques (ART) has become a common practice in human reproduction clinics. The preservation in liquid nitrogen (N2) at -196oC temperature means that the viability of the samples remains practically unchanged for years.
There is no unique method for cryopreserving sperm. The needs of a reproduction laboratory that cryopreserves samples from its patients with seminal pathologies are not the same as those of a sperm biobank, which has to offer its samples to centres that apply donor artificial insemination (IAD), in vitro fertilisation (IVF) or microinsemination sperm (ICSI) techniques with donor sperm.
Next, we will go through some sperm cryopreservation systems, considering that at the end of the cryogenic process, all of them end up storing the samples, submerged in liquid N2. We will focus on the support and freezing system rather than on the cryoprotective media (CPA).
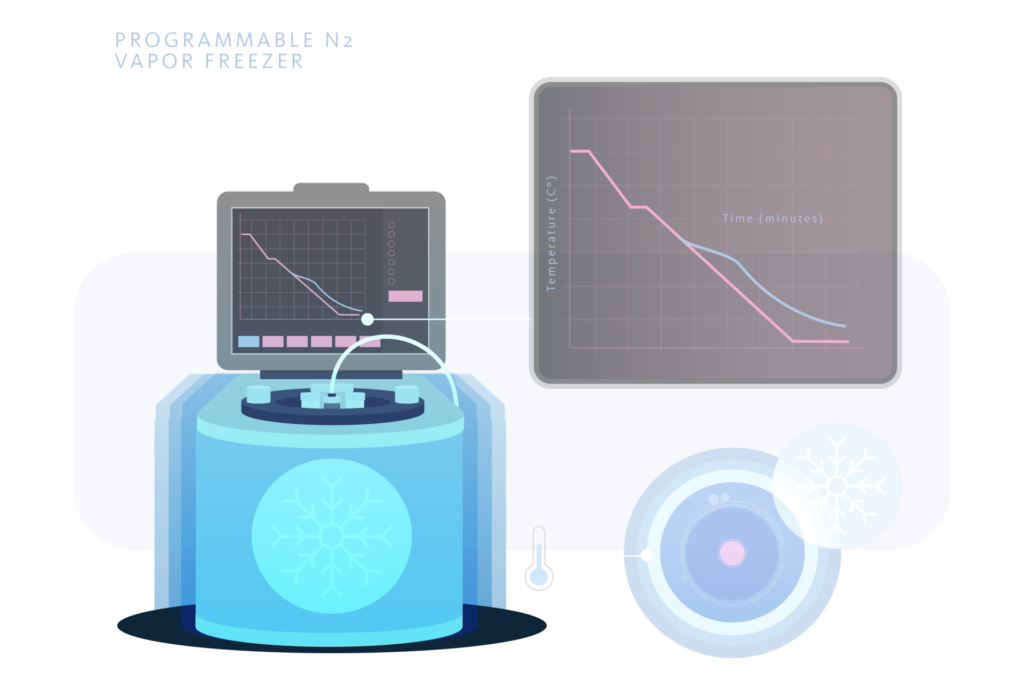
Programmable N2 freezer
Using a freezer connected to a computer, a freezing curve suitable for the type of cell to be cryopreserved can be programmed. In addition, this system allows the data of each freezing performed to be archived. These freezers usually include different types of freezing and thawing curves, as well as allowing the operator to develop his own curves with different cryogenic speeds. For all these reasons, the programmed slow freezing has a series of exceptional characteristics that we do not find in almost any other cryopreservation system. These characteristics are adaptability, traceability and reproducibility.
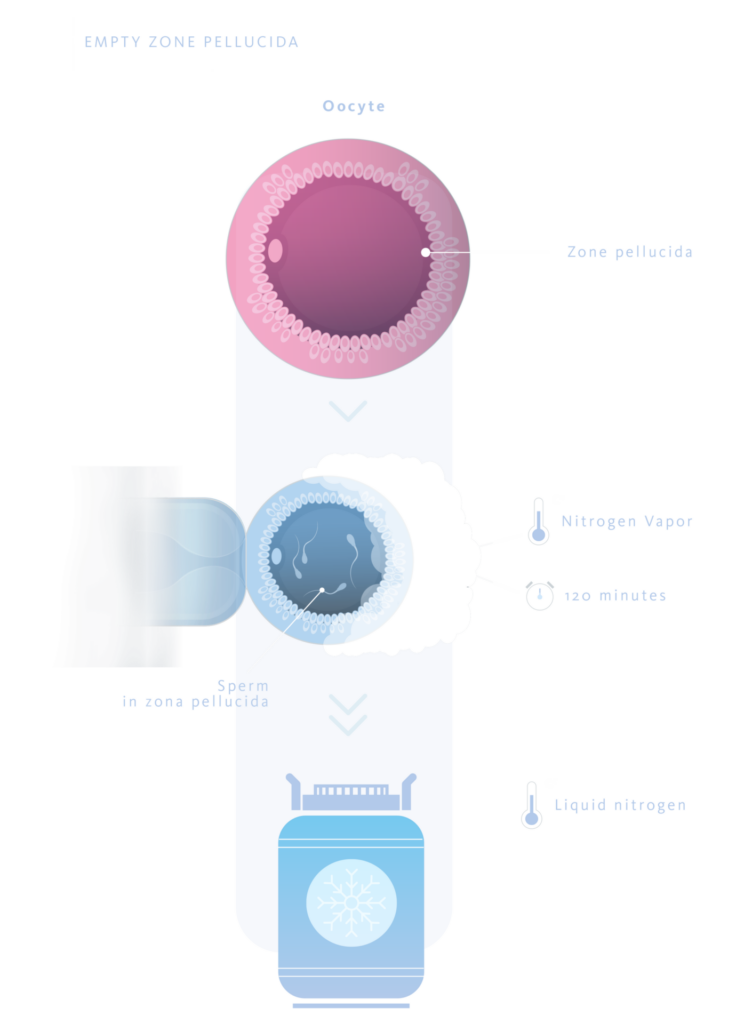
Empty zona pelucida
This system consists of using zona pellucida (ZP) from which the ooplasm has been removed, leaving only the ZP as a casing inside which the sperm to be frozen are deposited using micromanipulators.
The oocytes from which the ZP are obtained are human, immature (prophase or metaphase I) or unfertilized. Hamster and mouse oocytes can also be used, given their apparent morphological similarity to human oocytes.
The protocol defined by the authors (1) consists of loading the ZP with sperm in straws, keeping them in N2 vapors for 120 minutes. After this time, they are immersed directly in liquid N2. Using this technique, sperm recovery rates after thawing of 73% and fertilization rates of 50% were achieved, with live births reported.
The main international health agencies rejected the use of this technique due to the limited availability of oocytes, the ethical problems arising from using external oocytes and/or those from other animal species, as well as scientific evidence that traces of the original oocyte’s DNA could be preserved.
Volvox globator sphere
These chlorophycean algae are arranged in spherical colonies with a diameter between 0.5 and 1.0 mm and between 1,500 and 20,000 individuals. Since they have a shape and size similar to an oocyte, ICSI micromanipulators are used to insert sperm into them (about 8 sperm per sphere)(2).
A maximum of 3 spheres are loaded per 0.2 ml straw. These are then kept at 4ºC for 10 minutes. They then remain for another 10 minutes in N2 vapours and, finally, they are directly immersed in liquid N2. The sperm recovery and vitality rates after thawing were 100% and 60%, respectively(2).
Although sperm retrieval rates were unbeatable, this technique also faced ethical problems similar to those of empty zona pellucida. As a result, it was rejected by the main international health agencies without any live births being reported.

Hollow core agarose capsules
The sperm to be cryopreserved are microinjected into hollow spheres made from agarose with an external diameter of between 80 and 120 µm and an internal diameter of between 60 and 100 µm.
The spheres are made by mixing different streams based on sodium alginate, calcium carbonate, acetic acid, lecithin and vegetable oil. Using the microfluidics technique, the alginate gels and after going through a reaction channel and undergoing a subsequent cooling process, it is transformed into hollow agarose spheres(4).
After microinjecting one sperm per sphere, these are placed in a polycarbonate support with a size of 0.8-1.0mm. X 8-10mm. X 0.1mm. This support is heat-sealed to a straw, which makes it easy to handle. A maximum of 10 spheres are placed per support, in a CPA volume of 0.25 to 0.5 µl. Cryopreservation is carried out by keeping the support in N2 vapor at a height of 2 cm and between 10 to 30 seconds. Immersion is then carried out(4).
Although sperm survival rates after thawing were 81% (3) and 95%(4), there are no live newborns reported with this technique.
Hollow microcapsules of phenolic hydroxyl hyaluronan
Hydroxyl phenolic hyaluronan (HA-Ph) microspheres are generated using the co-fluid technique in which two fluids of different densities and at different speeds are slowly and pulsatilely projected through two tubes of different calibers. The outer tube, of larger diameter, contains liquid paraffin and hydrogen peroxide (H2O2). The inner tube, of smaller caliber, contains peroxidase and HA-Ph. After the action of the co-fluids and subsequent cooling, microcapsules are obtained with an external zone, analogous to the ZP, of HA-Ph gelled by the spatial reorganization experienced in its molecules because of the changes in bonds that accompany the action of peroxidase on H2O2. The core of the microcapsule remains in a gelatinous state, and it is necessary to degrade it with trypsin to leave the capsules hollow and to be able to introduce the spermatozoa.
A maximum of three spermatozoa are microinjected per HA-Ph microcapsule. These capsules have an average external diameter of 241.6+30.4 µm(5).
The microcapsules are vitrified using Cryotop as a support in 1.0 µl. of CPA based on 0.1M sucrose. They are kept in liquid N2 vapors at 4.5 cm. for 2 minutes and then submerged.
The sperm recovery and motility rates after thawing were 95% and 14%, respectively(5). There are no reported live newborns and although it presents fast sperm recovery times, it is a technique that is rarely used without clinical results to support it.

Cryoloops
System consisting of a nylon handle of 5.0 µl. volume. The handle is attached to an elongated handle that is held inside the screw cap of a cryotube in which the entire system is introduced.
It is used with CPA based on egg yolk and 12% glycerol, freezing in N2 vapors for 5 minutes at 3 cm from the liquidsurface and subsequent immersion, or by direct vitrification in N2. The rate of mobile sperm after thawing was 45% and no significant differences were observed between the two cryopreservation systems(6).
Another study(7) compared different cryopreservation systems, showing that the best results were obtained with direct vitrification without CPA and with programmed slow freezing with CPA based on egg yolk and 12% glycerol. The rate of spz. viable post-thawing was 52%. Fertilization rates in bovine research reached 67% and although this system has no ethical problems it has the disadvantage of being an open and unstable system with a risk of contamination and, having been little used, it lacks clinical results to support it.
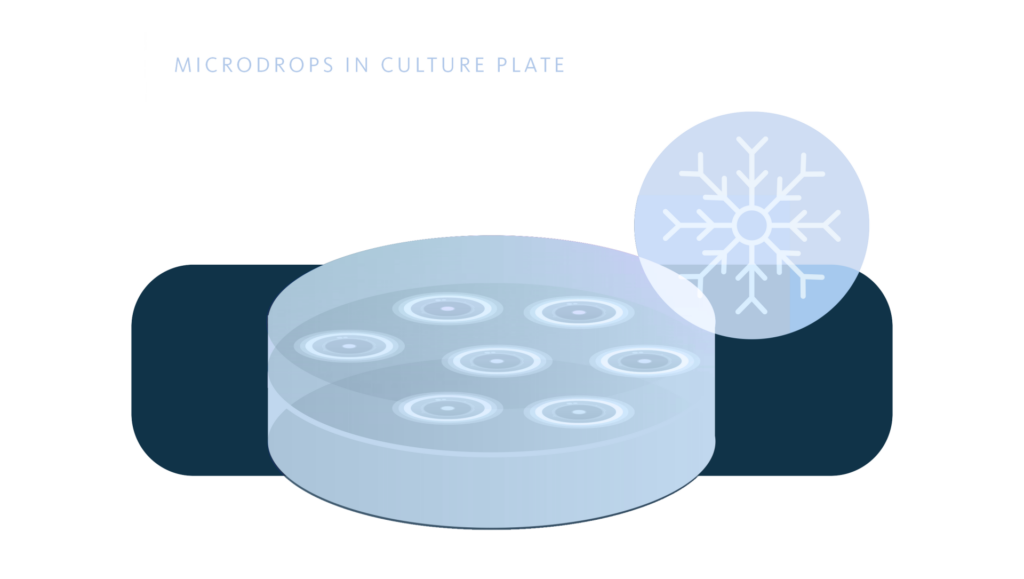
Microdrops in culture plate
Method that uses 60 mm diameter polystyrene plates as support, on which the spz. from testicular biopsy (8) are placed, in microdrops of 5.0 µl. volume with CPA based on 15% glycerol. The plate is then covered with 5.0 ml. of mineral oil, the plate lid is placed and it is introduced into a transparent plastic bag.
The bag with the plate is kept at a temperature of 4ºC for 15 minutes. It is then kept for 15 minutes in nitrogen vapors and finally immersed in liquid N2.
Although the rates of spz. recovered after thawing were 100%, the motility and fertilization rates were low (2 and 18% respectively). It is a very unstable, impractical technique, and with no reported live newborns, which greatly limits its clinical application.
Cell Sleeper
Closed system based on a plastic tray that is introduced into a screw-top polypropylene cryotube. The best results in sperm motility and vitality are achieved with 3.5 µl microdrops of CPA with 20% glycerol and without immersion in mineral oil(9). The ejaculated sperm or testicular biopsy sperm are loaded into the tray using ICSI micromanipulators. The tray is then introduced into the cryotube and closed with the screw-top cap, ensuring that the closure is not completely airtight to allow the passage of N2 into the interior and prevent possible explosions of the cryotube(9).
Vitrification is carried out by keeping the cryotube in N2 vapors, at a distance of 0.5 cm. for 2.5 minutes. After this time, it is immersed in liquid N2. The rates of spermatozoa are recovered after thawing in biopsy and ejaculate, were 83% and 100% respectively. In ejaculate spz., the fertilization rate was 83%. Although it is a closed, inert and easy-to-learn method and presents evolving pregnancies (>20 weeks)(10), it requires a long time to locate spz., there is a possibility of breakage of the micropipettes (given the shape of the tray) and it has few clinical cases.

Cryotop
Closed system consisting of a thin polypropylene strip attached to a plastic handle and a straw that protects the sample. It is used with micromanipulation methodology, placing the spz. in microdrops of 1-2 µl. of CPA. based on 20% glycerol (11).
Vitrification is carried out in N2 vapors at 2-4 cm. from the surface for 2 minutes and subsequent immersion in liquid N2.
It is a method that presents controversy regarding the type of CPA or whether the use or not of cryoprotectant in the process. It is also a widely tested method for its use in cryopreservation of oocytes and embryos. It is easy, clean, inert, closed and presents live newborn after devitrification of spz.

Cryoleaf
System consisting of a thin polystyrene strip and moistened cotton to ensure a wet atmosphere. The strip is loaded with the spz. by ICSI micromanipulators in microdrops of 0.2 µl. of CPA with 12% glycerol and 20% egg yolk(12).
Vitrification is carried out in N2 vapours at 1 cm. above the surface for 2 minutes and subsequent immersion in liquid N2.
The sperm motility rates after thawing in epididymal puncture and ejaculate were 93% and 62% respectively. Although it is a closed, clean and inert method, it requires speed to avoid drying of the medium, it has few cases, no lives newborn and it is no longer used.
Cryopiece
System consisting of a polystyrene strip (1x3cm) sterilized in ethylene oxide and introduced into a cryotube with a screw cap adapted to immobilize the strip. The ejaculated or biopsied sperm are loaded onto the strip using a micromanipulator and an ICSI plate covered with mineral oil to prevent evaporation of the medium. The microdrops are 2 µl of 12% glycerol-based CPA (13).
Vitrification is performed in N2 vapors at 5 cm. above the surface, for 10-15 minutes and subsequent immersion in liquid N2.
The sperm recovery and motility rates after thawing were 83% and 48%, respectively. It is a closed, inert, stable method and, although it is recent, it already has RNV, although it requires more clinical cases.
Closed slice
A system with very little information, consisting of a polypropylene strip (approx. 1x3cm) inserted into a cryotube with a screw cap. A single ejaculated or biopsied sperm is loaded per microdrop into each strip using a micromanipulator. When comparing the influences of microdrops medium volumes (0.5, 1.0 and 3.5 µl), as well as the use or not of CPA., no significant differences were found (14).
The cryotube is vitrified in N2 vapours at 1 cm above the surface for 2 minutes with subsequent immersion in liquid N2.
The sperm recovery and survival rates after thawing were 94% and 70% in ejaculated sperm and 92% and 66% in biopsied sperm. It is a method with a fertilization rate in biopsy sperm of 56%. It is a closed and inert method, but with very little information and clinical cases studied.
Sperm VD
It is a uniquely designed support made of 26x8x7 mm polycarbonate (medical grade) sterilized with gamma rays (15). It has three loading zones in which a microdrop (0.8 to 1.0 µl.) of vitrification medium is placed per zone. It is mounted on an ICSI plate with mineral oil and loaded by micromanipulator.
It is vitrified with a CPA based on sucrose, glucose and glycerol. Once the support is loaded with the spz, the excess oil is removed and it is introduced into a 3.6 ml. cryotube, which is not hermetically sealed to facilitate vitrification in N2 vapors at 1 cm. above the surface, for 2 minutes with subsequent immersion.
The recovery rates, motility, fertilization, pregnancy and delivery rates after thawing were 96%, 33%, 59%, 55% and 32%, respectively. Being a rapid spz. search system, inert, stable, closed and without ethical problems, it still needs more studies, as it has only recently appeared.
MQ Straws
This system is based on a 0.25 ml straw, cut into three equal parts of 4 cm. length and 60 µl. volume each one of them. The spz are loaded with 10 µl. of freezing medium in the straw sections using a Petri dish and a pipette. When compared different freezing media, the best results are with CPA based on 15% glycerol and 20% egg yolk (16).
Once the straw is loaded, the ends are heat-sealed and kept in N2 vapors at 5 cm. for 15 minutes. After this time, the straws are introduced into the cryotube and immersed in liquid N2(16).
It is a very recent method and, therefore, lacks sufficient case studies.
Vitrification
Vitrification is a physical process of converting a liquid medium into an amorphous solid similar to glass and lacking any crystalline structure, by mixing additives and rapid cooling. We can say that the main characteristics of vitrification are:
a) Very direct contact between sperm. and liquid N2.
b) High concentrations of CPA and a short exposure time.
c) Reduced vitrification time (2-10 minutes), which increases the simplicity of the process.
d) Very fast freezing speeds (15,000-30,000oC/min.).
There is a “non-aseptic” seminal vitrification system that consists of dropping the spz. in 30 µl drops of CPA directly into the liquid N2. After a few seconds, these drops are transformed into vitrified spheres that are placed in cryotubes that are stored in the storage cryocontainer (17).
Another system called “aseptic” consists of loading a 0.01 ml. dilution of spz. and CPA into a 0.25 ml straw. This straw is then introduced into another 0.5 ml straw which is heat sealed and introduced directly into liquid N2 (18).
It is important to note that, spontaneously, any freezing system in liquid N2 reaches the vitrification state around -140ºC.
BIBLIOGRAPHY
- Cohen J, Garrisi GJ, Congedo-Ferrara TA, et al. Cryopreservation of single human spermatozoa. Hum Reprod. 1997; 12(5):994-1001.
- Just A, Gruber I, Wöber M, et al. Novel method for the cryopreservation of testicular sperm and ejaculated spermatozoa from patients with severe oligospermia: a pilot study. Fertil Steril. 2004; 82(2):445-447.
- Isaev D, Zaletov S, Zaeva V, el al. Artificial microcontainers for cryopreservation of solitary spermatozoa. Hum Reprod. 2007; 22:i154.
- Araki Y, Yao T, Asayama Y, et al. Single human sperm cryopreservation method using hollow-core agarose capsules. Fertil Steril. 2015; 104(4):1004-9.
- Tomita K, Sakai S, Khanmohammadi M, et al. Cryopreservation of a small number of human sperm using enzymatically fabricated, hollow hyaluronan microcapsules handled by conventional ICSI procedures. J Assist Reprod Genet. 2016; 33:501-511.
- Schuster T, Keller L, Dunn R, et al. Ultra-rapid freezing of very low numbers of sperm using cryoloops. Hum Reprod. 2003; 18(4):788-795.
- Isachenko E, Isachenko V, Katkov I, et al. DNA integrity and motility of human spermatozoa after standard slow freezing versus cryoprotectant-free vitrification. Hum Reprod. 2004; 19(4):932-939.
- Sereni E, Bonu MA, Fava L, et al. Freezing spermatozoa obtained by testicular fine needle aspiration: a new technique. Reprod Biomed. 2008; 16(1):89-95.
- Endo Y, Fujii Y, Shintani K, et al. Simple vitrification for small numbers of human spermatozoa. Reprod Biomed Online. 2012; 24:301-307.
- Coetzee K, Ozgur K, Berkkanoglu M, et al. Reliable single sperm cryopreservation in Cell Sleepers for azoospermia management. Andrologia. 2015; 48:203-210.
- Endo Y, Fujii Y, Shintani K, et al. Single spermatozoon freezing using Cryotop. J Mamm Ova Res. 2011; 28:47-52.
- Peng QP, Cao SF, Lyu QF, et al. A novel method for cryopreservation of individual human spermatozoa. In Vitro Cell Dev Biol. 2011; 47:565-572.
- Sun J, Chen W, Zhou L, et al. Successful delivery derived from cryopreserved rare human spermatozoa with novel cryopiece. Andrology. 2017; 5:832-837.
- Ma C, Hu MG, Wang ZT. Study on ultra-rapid vitrification of human micro- sperm by closed-sheet method. Mod Obstet Gynecol Prog. 2015; 24:762-764.
- Berkovitz A, Miller N, Silberman M, et al. A novel solution for freezing small numbers of spermatozoa using a sperm vitrification device. Hum Reprod. 2018; 33:1975-1983.
- Ziarati N, Topraggaleh P, Rahimizadeh L, et al. Micro-quantity straw as a carrier for cryopreservation of oligozoospermic semen samples: Effects of storage times and cryoprotectant. Cryobiology 2019; 86: 65-70.
- Isachenko E, Isachenko V, Weiss, JM, Kreienberg R, Katkov II, Schulz M, Lulat AG-M, Risopatrón MJ and Sánchez R. Acrosomal status and mitocondrial activity of human spermatozoa vitrified with sucrose. Reproduction. 2008; 136: 167-173.
- Isachenko V, Isachenko E, Montag M, Zaeva V, Krivokharchenko I, Nawroth F, Dessole S, Katkov II and Van der Ven H. Clean technique for cryoprotectant-free vitrification of human spermatozoa. Reprodutive BioMedicine. 2005; 10(3): 350-354.