Sleep quality and male fertility: what does science say?
Infertility is a common reproductive health problem, affecting approximately 15% of couples worldwide. The male factor contributes to 20–70% of infertility cases, with male infertility prevalence estimated between 2.5% and 12% of the global male population. In recent years, lifestyle factors such as sleep quality and sleep duration have gained attention as potential contributors to male reproductive health.
Although research on the relationship between sleep and infertility is still limited, several studies have shown a clear association between poor sleep quality, short or excessive sleep duration, and impaired sperm parameters. Men who experience difficulty falling asleep, fragmented sleep, or chronic sleep deprivation tend to present lower sperm volume and reduced sperm motility, two key markers of semen quality.
Evidence linking sleep quality to sperm parameters
A cross-sectional study conducted in male partners of infertile couples found that sleep quality had a statistically significant effect on sperm concentration, while sleep duration significantly influenced sperm motility. No clear association was observed with sperm morphology, suggesting that sleep may primarily affect sperm production and movement rather than structure. However, the authors highlighted the need for further studies with larger and more diverse populations to confirm these findings.
Additional evidence comes from a large study involving 842 healthy men screened as potential sperm donors, in which repeated semen measurements were analyzed. Using more than 5,600 semen samples, researchers observed that both short sleep duration (less than 6 hours per day) and long sleep duration (more than 9 hours per day) were associated with lower sperm volume and reduced total and progressive motility. Men reporting poor sleep quality also showed lower total sperm count and decreased motility compared to those with good sleep quality. These findings suggest that both insufficient and excessive sleep may negatively affect male fertility.
Poor sleep quality, hormones, and reproductive potential
Further research involving 970 male outpatients undergoing fertility evaluation showed that poor sleep quality was associated with lower sperm concentration, total sperm count, motility, and normal sperm morphology. Interestingly, no significant association was found between sleep quality and reproductive hormone levels, indicating that the relationship between sleep and male fertility may involve non-hormonal mechanisms, such as oxidative stress or cellular damage.
The clinical relevance of sleep quality is also evident in assisted reproductive treatments. A retrospective study analyzing 282 subfertile couples undergoing ART procedures found that better male sleep quality was positively associated with higher fertilization rates, increased birth weight, and higher live birth rates, highlighting the potential impact of male sleep health on treatment outcomes.
Digital screens, sleep disruption, and sperm quality
Modern lifestyle habits may further exacerbate sleep-related fertility issues. Studies evaluating evening and post-bedtime exposure to light-emitting digital screens have shown a negative association with sperm quality. Smartphone and tablet use at night has been linked to lower sperm concentration, reduced total and progressive motility, and a higher percentage of immotile sperm. In contrast, longer sleep duration was positively correlated with sperm motility, reinforcing the importance of healthy sleep habits for male reproductive health.
Biological mechanisms and long-term consequences
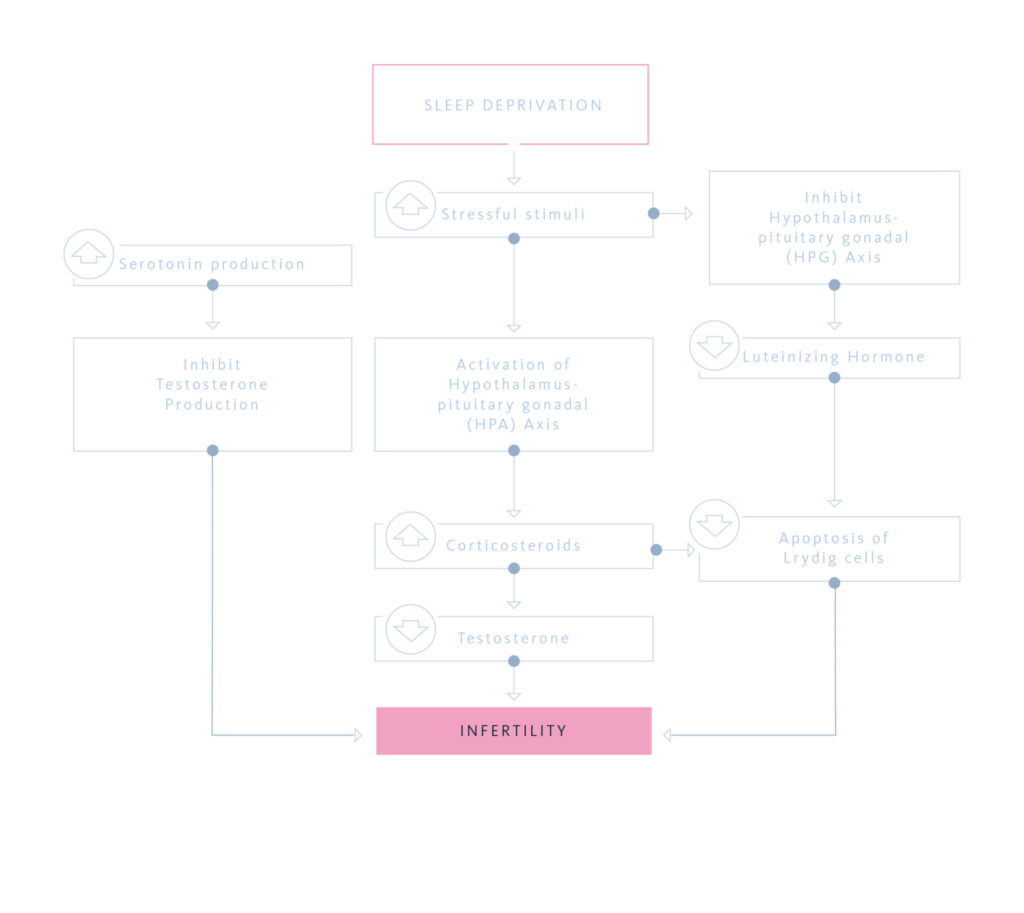
Experimental and clinical evidence suggests that sleep deprivation induces physiological changes similar to oxidative stress, activating the hypothalamic–pituitary–adrenal (HPA) axis while inhibiting the hypothalamic–pituitary–gonadal (HPG) axis. This imbalance leads to increased corticosteroid levels, which have been implicated in both male and female infertility. Circadian rhythm disruption, such as that caused by shift work, further affects reproductive health by altering the regulation of sex steroids, gonadotropins, and prolactin.
Emerging data also indicate that the effects of sleep deprivation may extend beyond the individual. Poor sleep during critical periods, including pregnancy, may have transgenerational consequences, potentially impairing reproductive function and sexual motivation in offspring.
Conclusion
Current scientific evidence suggests that sleep quality, sleep duration, and circadian rhythm stability play a meaningful role in male fertility. Poor sleep is associated with impaired sperm parameters, reduced reproductive potential, and poorer outcomes in assisted reproduction. Promoting healthy sleep habits should therefore be considered an important component of male reproductive health, while further large-scale and mechanistic studies are needed to fully clarify this relationship.
References
- 1. Viganò P, Chiaffarino F, Bonzi V, Salonia A, Ricci E, Papaleo E, Agnese Mauri P and Parazzini F. Sleep disturbances and semen quality in an Italian cross sectional study. Basic Clin Androl. 2017; 27: 16. DOI 10.1186/s12610-017-0060-0.
- 2. Budihastuti UR, Melinawati E, Prakosa T, Angelia Ratnasari A, Hadi C, Laqif A, Pangestu M, Oktadiani Putri L, Murti B and Nurwati I. Influence of age, obesity, smoking, sleep duration and sleep quality on concentration, morphology, and sperm motility: a cross-sectional study. Int J Fertil Steril. 2024; 18(3): 240-247. DOI 10.22074/IJFS.2023.1983273.1413
- 3. Heng-Gui Chen, Bin Sun, Ying-Jun Chen, Jorge E. Chavarro, Si-Heng Hu, Cheng-Liang Xion, An Pan, Tian-Qing Meng, Yi-Xin Wang and Carmen Messerlian. Sleep duration and quality in relation to semen quality in healthy men screened as potential sperm donors. Environment International. 2020; 135. DOI .org/10.1016/j.envint.2019.105368.
- 4. Cong-Qi Du, Yo n g – Y i Ya n g, Jing Chen, Lei Feng and Wen-Qin Lin. Association Between Sleep Quality and Semen Parameters and Reproductive Hormones: A Cross-Sectional Study in Zhejiang, China. Nature and Science of Sleep. 2020; (12): 11–18.
- 5. Cong-Qi Du, Dong-Xue Zhang, Jing Chen, Qiu-Fen He and Wen-Qin Lin. Men’s Sleep Quality and Assisted Reproductive Technology Outcomes in Couples Referred to a Fertility Clinic: A Chinese Cohort Study. Nature and Science of Sleep. 2022; (14): 557-566.
- 6. Amit Green, Shlomi Barak, Lior Shine, Arik Kahane and Yaron Dagan. Exposure by males to light emitted from media devices at night is linked with decline of sperm quality and correlated with sleep quality measures, Chronobiology International. 2020; DOI 10.1080/07420528.2020.1727918.
- 7. Lateef OM and Akintubosun MO. Sleep and Reproductive Health. Journal of Circadian Rhythms. 2020; 18(1): 1, pp. 1–11. DOI https://doi.org/10.5334/jcr.190.
- 8. Li T, Bai Y, Jiang Y, Jiang K, Tian Y, Gu J and Sun F. The potential impacts of circadian rhythm disturbances on male fertility. Front. Endocrinol. 2022; 13:1001316. DOI 10.3389/fendo.2022.1001316