Efficacy of patient’s friendly ovarian stimulation
Over the years, various protocols of ovarian stimulation have been employed, with the underlying principle being the induction of multifollicular development and the prevention of spontaneous ovulation. In recent years, the development of new protocols with the aim of reducing the impact of assisted reproductive treatments on patients has led to the so-called progestin-primed ovarian stimulation (PPOS) becoming a popular approach (R. Rodríguez et al)
The use of new ovarian stimulation protocols, starting during the luteal phase or randomly shows that endogenous progesterone alone is sufficient to block the LH surge and does not compromise oocyte competence and pregnancy outcomes. For these reasons, exogenous progesterone was utilized as an innovative model of pituitary suppression
The evidence demonstrates that the use of Medroxiprogesterone acetate (MPA) during ovarian stimulation is effective in blocking the LH surge and does not affect the number of oocytes collected or the quality of the embryos obtained. To date, PPOS protocol has been successfully employed in patients with polycystic ovary syndrome, poor ovarian response, oocyte donors, fertility preservation cycles, and the efficacy and efficiency of the MPA does not seem to be affected by the type of gonadotropin used (S. Bontá et al)
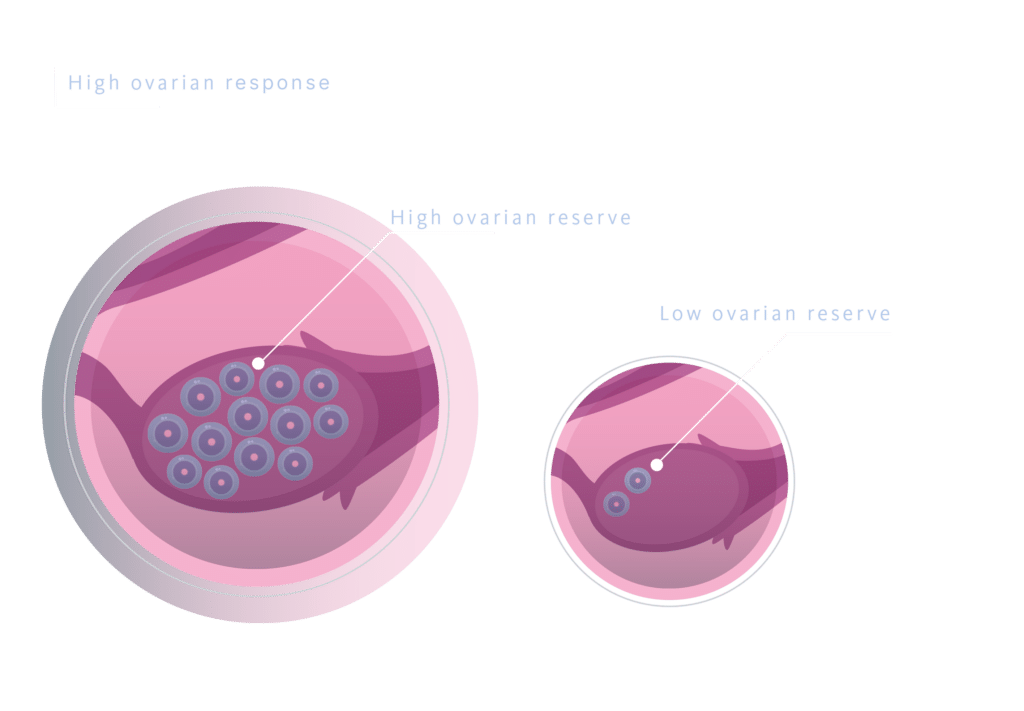
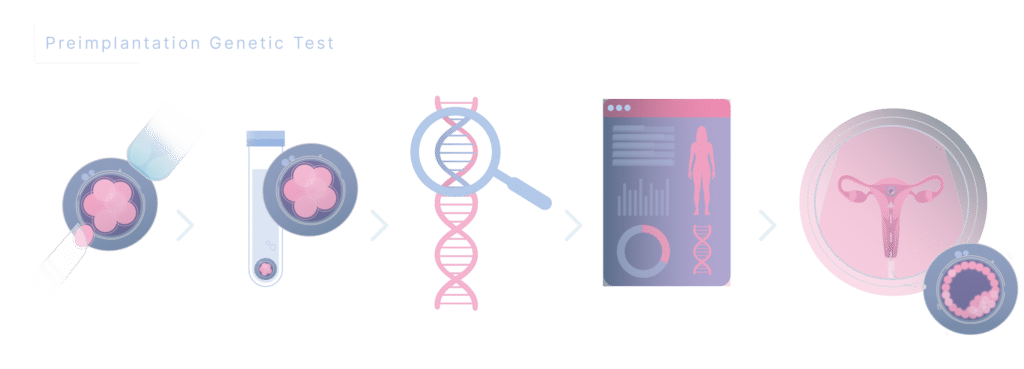
This protocol is designed for patients in whom a fresh embryo transfer is not planned or possible due to high ovarian response, treatments of ovarian preservation, or in cases where a preimplantation genetic test is planned. The benefits of this protocol include an easier administration (oral instead of subcutaneous), greater cost-effectiveness with the same efficacy as previous protocols, which increases compliance with treatment and reduces the risk of dropout.
Flexible PPOS
Recently there has been a suggestion that the administration of the PPOS protocol could be made flexible. The study by Yildiz S. represents one of the earliest assessments of the effectiveness of a progestin administered later in the stimulation cycle. This approach is designated as “flexible progestin primed ovarian stimulation” (f PPOS), in comparison to a flexible GnRH antagonist protocol. The authors concluded that f PPOS yielded significantly more total oocytes and more metaphase II oocytes than flexible GnRH antagonist cycles (24 [17–34] vs. 21 [15-28], respectively; P < 0.01), with similar gonadotropin consumption and duration of stimulation. Theoretically, the absence of pituitary suppression at the early follicular phase and the possible less profound suppression with progestins relative to GnRH agonist at the late follicular phase could have resulted in the collection of a greater number of oocytes with the f PPOS protocol.
Design, description and results of our clinical study
The objective of our research was to assess the effectiveness of the flexible PPOS protocol, starting the administration of progestin (MPA) in a later phase of the ovarian stimulation cycle, comparing its results with the use of MPA, starting in a traditional way at the first day of ovarian stimulation.
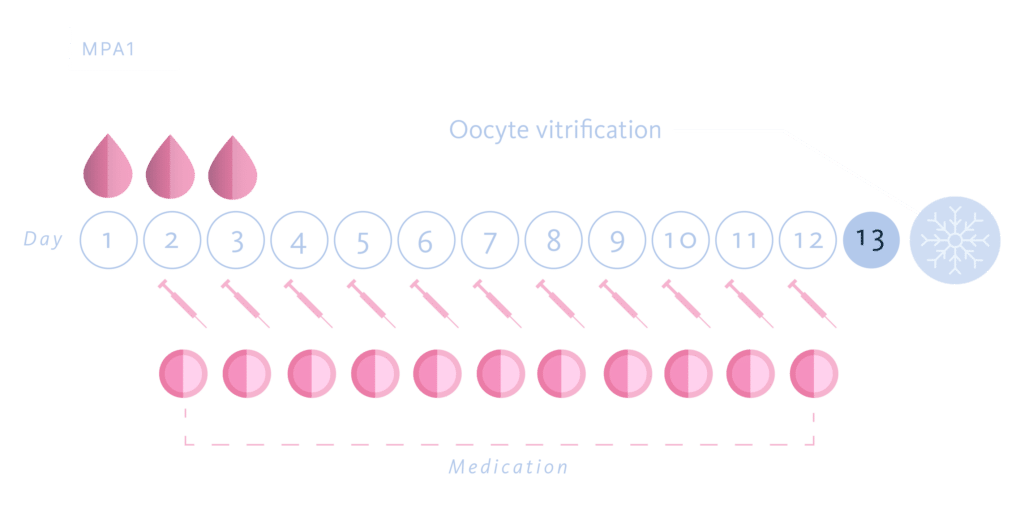
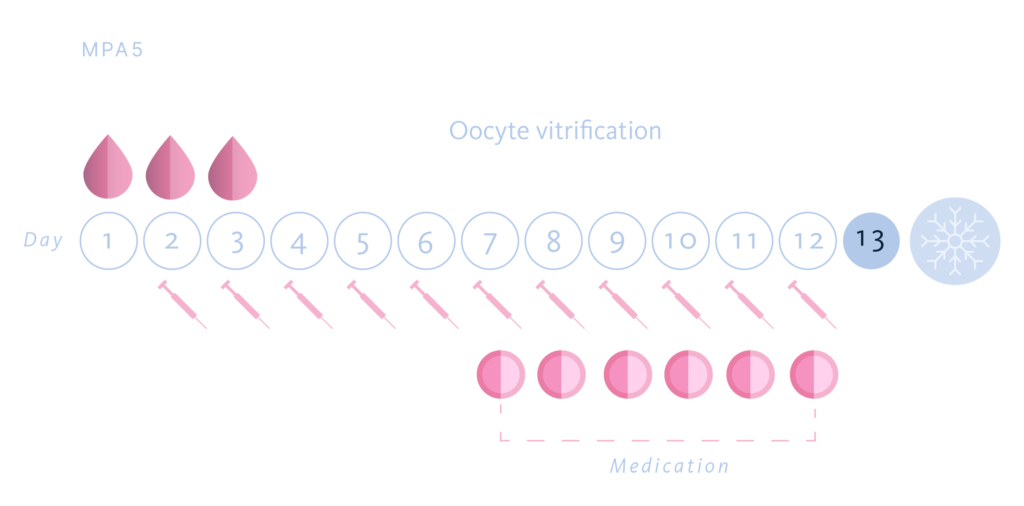
For which we designed a multicenter, prospective, randomized, paired study. 85 patients were recruited and underwent two consecutive cycles of ovarian stimulation for oocyte donation. The patients were divided into two groups, MPA Group 1: start of MPA on the first day of stimulation and MPA Group 5: start of MPA on the fifth day of ovarian stimulation. In total, 170 cycles were carried out. The choice of the start day of MPA in each cycle for each patient was done randomly, using a randomization tool.
The oocyte donors were healthy women aged 18–35 years with regular menstrual cycles, a BMI of 18–29 kg/m2 and have no contraindications or allergies to medicaments used for controlled ovarian stimulation. We excluded woman with anormal karyotype or hereditary X-linked diseases, sexually transmitted diseases and a poor ovarian reserve characterized by an antral follicle count of < 12 follicles in both the ovaries and an anti-müllerian hormone (AMH) level of < 2,5 ng/mL at the beginning of the cycle.
In all cases we used the same dose and the same gonadotropin in the donor’s two donation cycles (FSH-r 225 UI/day) and depending on the randomly assignment group we started 10 mg/day of MPA either the first day of COH (group MPA 1) or the fifth of COH (group MPA 5.) When at least four or more follicles reach a diameter ≥ 17 mm, a GnRH agonist, Triptorelin (Decapeptyl®) was administered for the trigger in both groups, in a single dose of 0.2 mg. Transvaginal oocyte retrieval was performed 36 hours after triggering.
The main outcome was comparing the oocyte yield calculated as the number of total oocytes, of mature oocytes (MII) and of useful MII oocytes (no dysmorphic MII oocyte) collected. Secondary outcome was compared the duration of COH, the total dose of gonadotropin used, and cancellation rate.
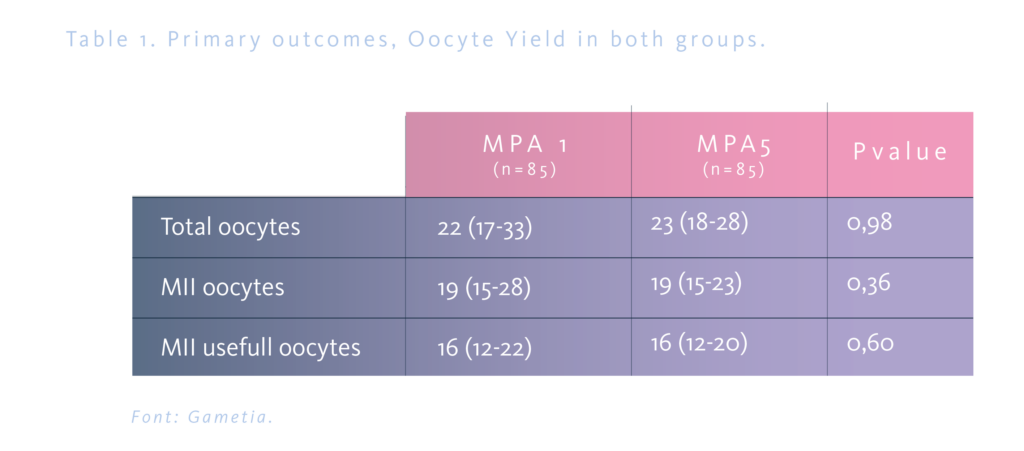
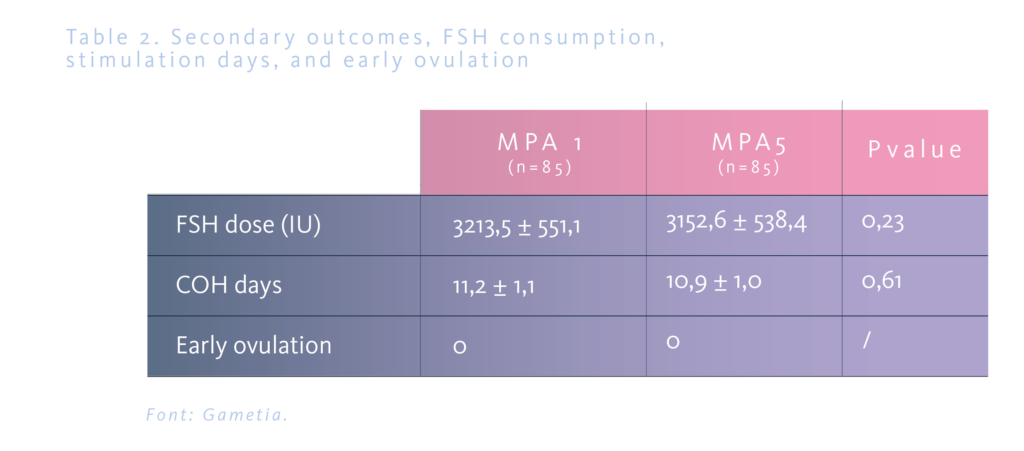
The total number of oocytes was 22 in the MPA1 group and 23 in the MPA5 group, without statistical significance; the number of MII and MII usefull was 19 and 16 oocytes respectively in both groups (Table 1). The mean duration of the cycle was similar in both groups, 11.2±1.1 and 10.9±1.0 days, respectively, as well as the total consumption of FSHr (3213.5±551.1 and 3152.6±538.4 IU). In both groups, 2.3% of the cycles were cancelled, and none of the cases were related to early ovulation (Table 2).
Patient satisfaction and treatment efficacy can be achieved
The effectiveness of assisted reproduction treatments is one of the most important factors when determining the most appropriate therapeutic plan. The combination of protocols that allows greater efficacy with greater compliance, simplicity, convenience and economy, represent important aspects of modern medicine. Therefore, it is imperative to persist in researching alternative methods to achieve this result and provide our patients with the most optimal care.
References
- Rodríguez Martín, Rocío; Cabrera Rodríguez, Desirée; Romero Matas, Marta; Mora Palma, José Carlos; Diaz Giráldez, Rocío; Bonta, Silvia, Gonzalez Utor Antonio Luis, Quintero Espinel Luis Alberto. Dos modelos de supresión hipofisaria en protocolo de estimulación ovárica combinado: Estudio pareado en donantes de ovocitos. 33º Congreso Nacional Sociedad Española de Fertilidad, 2022.
- Silvia Bontà1, Antonio Forgiarini1, Sara Maggi1, Greet Lammens1, Inmaculada Navarro1, Alba Burguera1, Luis Quintero1. Analysis of the oocyte yield and ovarian sensitivity index with the use of two gonadotropins (fshr/fshu) and two pituitary suppression protocols (PPOS/GnRH-ant). Human Reproduction, ESHRE’23 abstract book, 2023.
- Sule Yildiz, Engin Turkgeldi, Berk Angun, Alper Erasian, Bulent Urman, Baris Ata. Comparison of a novel flexible progestin primed ovarian stimulation protocol and the flexible gonadotropin-releasing hormone antagonist protocol for assisted reproductive technology. Fertility & Sterility, 2019.
