PPOS protocol as “Gold Standard” in oocyte donation programs
A crucial element during ovarian stimulation is preventing premature follicular ovulation. For decades, GnRH analogues have been used for this purpose. Among the various disadvantages of using these drugs is the limited flexibility. Additionally, high costs and their subcutaneous administration may be inconvenient for patients.
Studies on the spontaneous suppression of the LH surge in the luteal phase during random-start cycles have led to the idea of using exogenous progestins to prevent premature ovulation during ovarian stimulation, giving raise to the so called progestin-primed ovarian stimulation (PPOS) protocol,
Many studies demonstrated a same efficacy and efficiency as the “classic” protocols with GnRH analogues, regardless of the type of gonadotropins used, the day of starting progesterone administration, and the type of progesterone used. Given the reassuring results obtained, an increasing number of assisted reproduction centers have implemented its use due to its multiple advantages. Firstly, the oral administration of the drug is significantly less traumatic for patients compared to traditional subcutaneous injections. This aspect is particularly useful in patient groups like egg donors, where ensuring simple and “friendly” standard protocols is crucial for an adequate compliance. Another important aspect is the significant reduction in procedure costs, approximately 10-20 times lower than conventional cycles with GnRH antagonists. However, this type of protocol can only be used in cases where a freeze-all approach is employed, such as fertility preservation, preimplantation genetic test, high-risk of ovarian hyperstimulation syndrome (OHSS) cases, “non-conventional” stimulation cycles like random start, Duo-Stim cycles, and, of course, egg donation cycles.
For these reasons, the PPOS protocol has become the gold standard for pituitary suppression in oocyte donors.
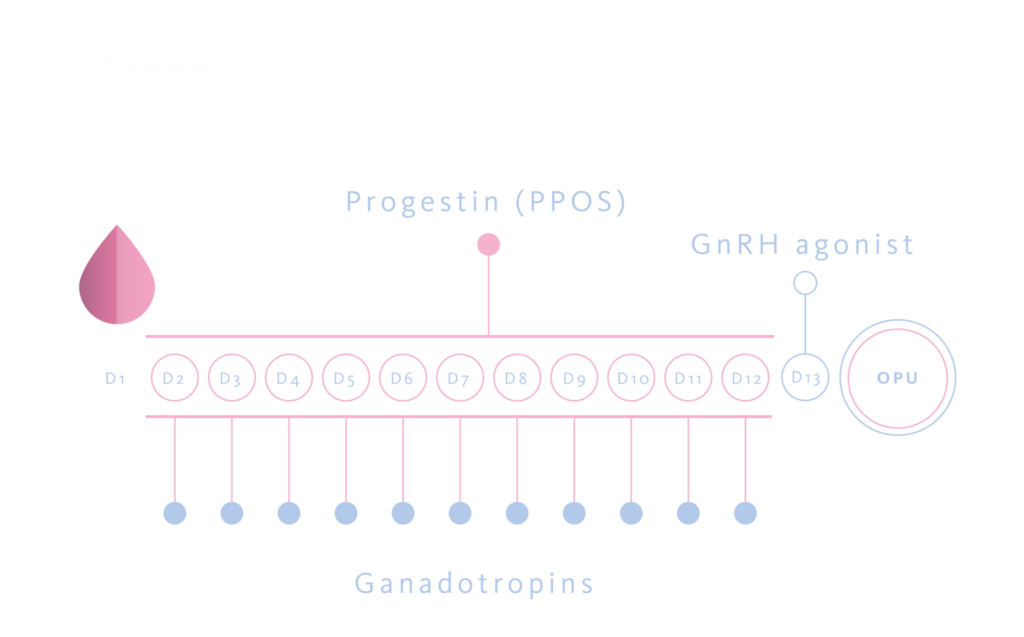
Stardard of care in an egg donation program
In a egg donation program, donors usually undegone to multiple controlled ovarian hyperstimulation (COH) cycles with at least a 3 months interval. Concerns about possible negative effects on ovarian response and safety of repeated COH cycles in the same patient have been raised, but it has been proven that performing multiple cycles in the same patient with classic pituitary suppression protocols using GnRH-analogues (long GnRH-agonist protocol, short GnRH-antagonist protocol etc.) does not reduce the number or quality of oocytes retrieved or represents a risk for patients and the reproductive outcomes.
On the other hand, could using a PPOS protocol in multiple repeated cycles have a negative effect on oocyte yield and patient’s safety? Nowdays, data in literature are lacking. For these reasons, a study evaluating whether the ovarian response of oocyte donors after COH remains consistent or varies in successive cycles was realized in our centers.
Description, design and result of our clinical study
328 COH cycles were analyzed, performed in 126 donors. These included 126 first cycles, 126 second cycles, 54 third cycles, and 22 fourth cycles. In all cases, a PPOS protocol were used with medroxyprogesterone acetate 10 mg/day orally. For COH a subcutaneous recombinant FSH alone was preferred, at a fixed daily dose of 225 UI or 300 UI depending on antral follicle count or previous cycle’s results. A GnRH-agonist (triptorelin 0,2 mg subcutaneous) was administered 36 hours before the pick-up as ovulation trigger.
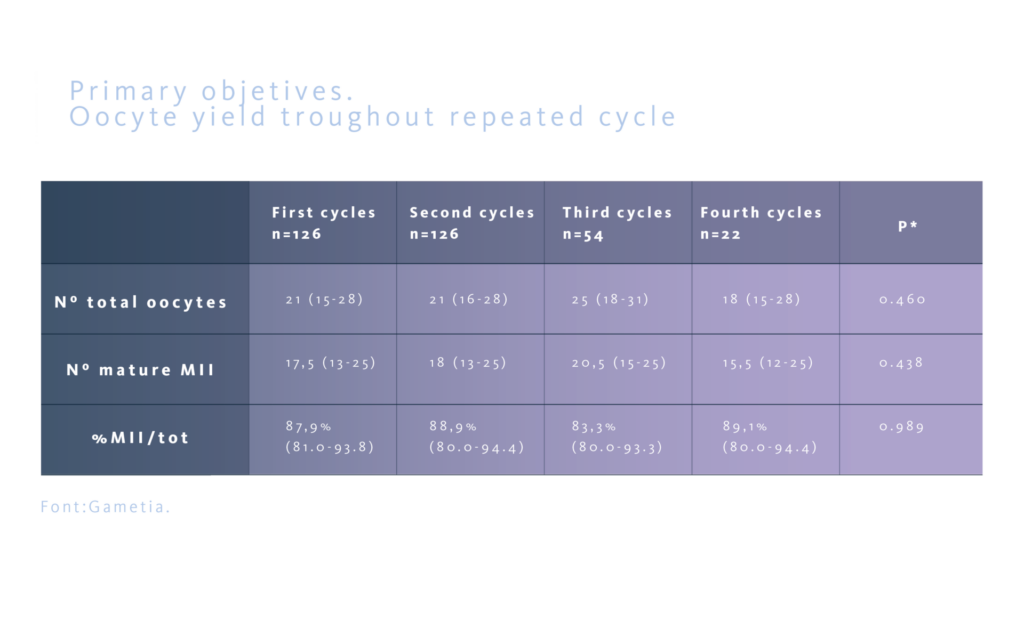
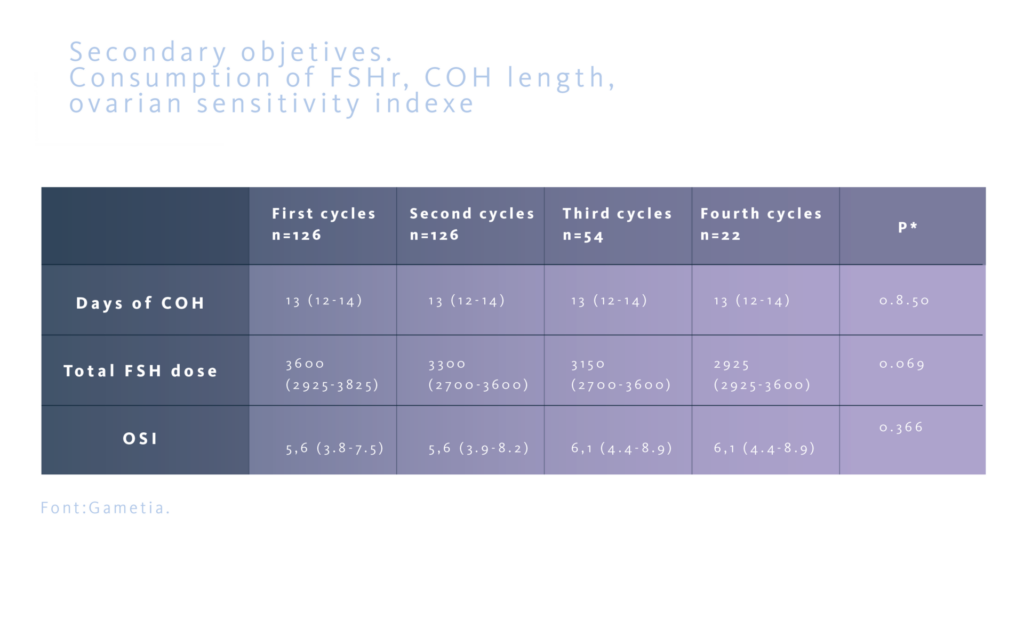
The study assessed the oocyte yield, defined as the number of total oocyte retrived and the number of mature MII oocytes, and the precentage of MII oocytes as primary objectives. Secondly, the ovarian sensitivity index (OSI), defined as the number of MII oocytes for every 1000 UI of FSHr used, the total stimulation days, and the total dose of FSHr used, were evaluated. The aim was to confirm that the results maintain similar troughout subsequent cycles in the same donor.
No statistically significant differences were observed between the different groups regarding the total number of oocytes retrieved and the number of mature MII oocytes , as well as in the percentage of MII oocytes/total oocytes obtained.
In all groups, the number of stimulation days and the total FSHr dose used were similar. The ovarian sensitivity index also showed similar results across all groups.
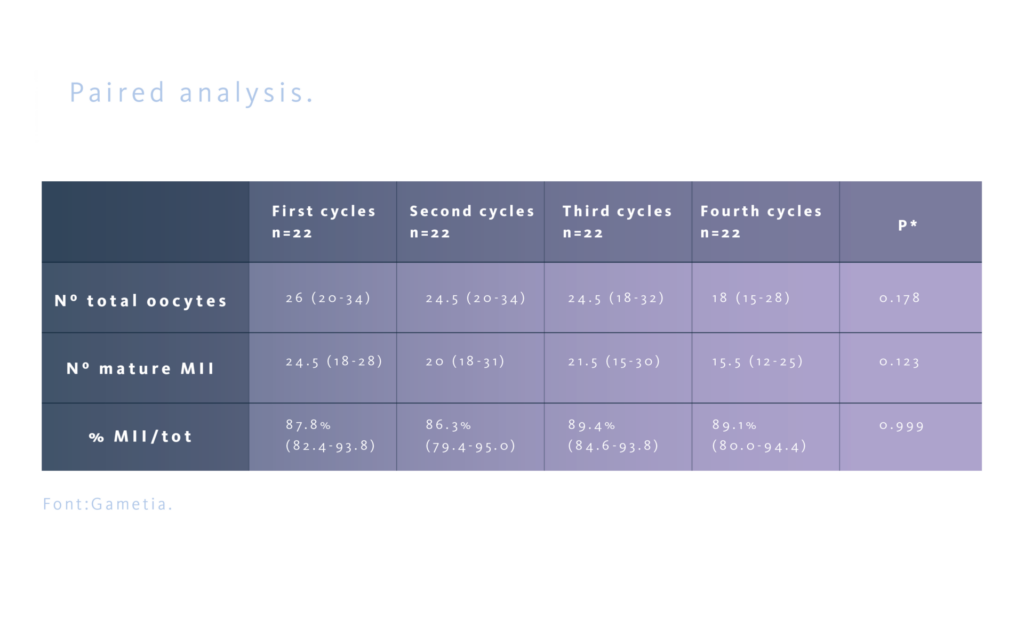
Even if egg donors represent a homogeneous group of young women (aged 18-35), not infertile, healthy, with a BMI of 18-29.9 m²/kg, to further support these results, a comparative paired analysis was conducted on the 22 donors who completed four consecutive cycles, thereby eliminating potential biases due to interpersonal differences and confirming the results obtained in the overall population.
The PPOS protocol garantees safety and efficiency troughout at least four repeated controlled ovarian hyperstimulation cycles
PPOS protocols do not seem to have any adverse effect on ovarian response, as on quality of oocytes collected, at least across four repeated cycles, and it could be considered safe for the patient. The oocyte yield of the first cycle maintain similar across successive cycles, so it can be considered predictive of ovarian response in subsequent cycles in oocyte donors. These results can be extended to groups of patients with similar characteristics, such as in fertility preservation programs. Different results in the group of infertile patients (for example, those with advanced maternal age) could be more related to the specific patients’ characteristics rather than the protocol used.
Bibliography
- Ata, B., Capuzzo, M., Turkgeldi, E., Yildiz, S., & La Marca, A. (2021). Progestins for pituitary suppression during ovarian stimulation for ART: A comprehensive and systematic review including meta-analyses. In Human Reproduction Update (Vol. 27, Issue 1, pp. 48–66). Oxford University Press. https://doi.org/10.1093/humupd/dmaa040
- Martinez, F., Racca, A., Rodríguez, I., & Polyzos, N. P. (2021). Ovarian stimulation for oocyte donation: a systematic review and meta-analysis. Human Reproduction Update, 1–24. https://doi.org/10.1093/humupd/dmab008
- Sule Yildiz, Engin Turkgeldi, Berk Angun, Alper Eraslan, Bulent Urman, Baris Ata. Comparison of a novel flexible progestin primed ovarian stimulation protocol and the flexible gonadotropin-releasing hormone antagonist protocol for assisted reproductive technology. Fertil Steril. 2019 Oct;112(4):677-683. doi: 10.1016/j.fertnstert.2019.06.009. Epub 2019 Jul 29.
- C. Caligara, J. Navarro, G. Vargas, C. Simon, A. Pellicer, J. Remohi. The effect of repeated controlled ovarian stimulation in donors. Hum Reprod. 2001 Nov;16(11):2320-3. doi: 10.1093/humrep/16.11.2320.
- L.T. Paul, O. Atilan and P. Tulay. The effect of repeated controlled ovarian stimulation cycles on the gamete and embryo development. Zygote. 2019 Oct;27(5):347-349. doi: 10.1017/S0967199419000418. Epub 2019 Aug 13
